Coal Carbonization for Coke Production
Coal Carbonization for Coke Production
Coal carbonization is the process by which coal is heated and volatile products (liquid and gaseous) are driven off, leaving a solid residue called coke. Carbonization of coal involves heating coal to high temperatures either in the absence of oxygen (O2) or in control quantity of O2. A gaseous by-product referred to as coke oven gas (COG) along with ammonia (NH3), water, and sulphur compounds are also thermally removed from the coal. The coke which remains after this distillation largely consists of carbon (C), in various crystallographic forms, but also contains the thermally modified remains of various minerals which have been in the original coal. These mineral remains, usually referred to as coke ash, do not burn and are left as a residue after the coke is burned.
Until recently, the carbonization of coal was considered as ‘destructive distillation’, but with the increased importance of the products of carbonization, this phrase is falling out of use. Now, the coal carbonization is considered to be a physico-chemical process which depends on the coking rate, operating parameters, coal blend properties and the transport of thermal energy. The heating rate of coal influences the strength and the fissuring properties of coke. In order to arrive at a homogeneous quality, the heating of the coal cake in a coke oven is therefore to be uniform over the total length and height of the oven. In addition to this, the plastic layer migration rate influences the level of thermal stress in the re-solidified mass and therefore, the level of fissuring.
The coal carbonization process started at the beginning of the 18th century by carbonizing good quality of coking coal in heaps on the ground, which subsequently led to the development of beehive ovens of different shapes and sizes to meet the increasing demands of hard coke needed for the smelting of iron.
Carbonization of coal can be carried out at the following three temperature ranges.
- Low temperature carbonization is normally carried out in the temperature range of 500 deg C to 700 deg C. In this type of carbonization, the yields of liquid products are higher and there is lower gaseous product yield. The coke produced is having higher volatile matter (VM) and is free burning.
- Medium temperature carbonization is done at temperature range of around 800 deg C. This carbonization produces smokeless soft coke. By-products produced are similar in characteristics to high temperature carbonization. Medium temperature carbonization is rarely practiced these days.
- High temperature carbonization is carried out at a temperature which is above 900 deg C. This carbonization gives higher yield of gaseous products and lower yield of liquid products. This carbonization produces hard coke from coking coals.
Low temperature carbonization
Low temperature carbonization was originally developed for providing town gas for residential and street lighting and for the production a smokeless fuel for domestic and industrial heating. The by-product tars are economically important and are often essential feed stocks for the chemical industry or are refined to gasoline, heating oils, and lubricants. The preferred coals for low temperature carbonization are typically lignite coal, subbituminous coal, or highly volatile bituminous coal, which, when pyrolyzes in the temperature range of 500 deg C to 700 deg C, yield a porous char with reactivities which are typically not much lower than those of their parent coals. These reactive chars (i) are easily ignited and are used as smokeless fuels or as feed stocks to gasification processes, (ii) are blended with coals to make coke oven feed, or (iii) are used as a power plant fuel.
The tars which are produced during low temperature carbonization are much different than those from high temperature carbonization. High temperature carbonization tends to produce mainly aromatic compounds, whereas those produced during low temperature carbonization are predominately aliphatic compounds, hence the different end use applications of the tar by-products. Gas yield and composition are also different during low temperature carbonization, with gas yields being around 25 % of those produced during high temperature carbonization, but the gas contains more methane (CH4) and less hydrogen (H2), giving it a higher heating value .
The main application of low-temperature carbonization is to make smokeless fuels for use in homes and small industrial boilers in areas which have high population density and rely on coal as a fuel, particularly coal which has high VM content.
High temperature carbonization
The main purpose of high temperature carbonization is the production of metallurgical coke for use in blast furnace (BF) and foundry. Some coke is used for the production of calcium carbide (CaC2) and electrode carbons. More than 90 % of the coke produced is used in BFs to smelt iron ore for the production of hot metal.
There are three types of processes for the high temperature carbonizing of coal. These are (i) beehive ovens, (ii) by-product recovery coke ovens, and (iii) non-recovery/ heat-recovery coke ovens.
The beehive oven is a simple domed brick structure into which coal can be charged through an opening at the top and then leveled through a side door to form on a bed of around 600 mm to 900 mm thick. Heat is supplied by burning the VM released from the coal, and carbonization progresses from the top down through the charge. Around 5 tons to 6 tons of coal can be charged, and a period of 48 hours to 72 hours is needed for the carbonization. Some beehive ovens are still in operation because of system improvements and the addition of waste heat boilers to recover heat from the combustion products.
The first by-product recovery coke ovens which produced satisfactory BF coke or foundry coke as the main product, and tar, ammonia, and later benzene as by-products, were built around 1856. Modifications to the design have continued but the basic design of these ovens, essentially the modern coke oven, was completed by the 1940s. The horizontal slot-type coke (by-product recovery) oven, in which higher temperatures can be attained and better control over coke quality can be exercised, has superseded other designs and is used for coking bituminous coal.
Modern by product coke ovens are comprised of chambers 15 metres (m) to 20 m long, 6 m to around 9 m high, 500 mm to 600 mm wide and having a wall thickness of around 100 mm. A number of these chambers (from 20 to 100) alternating with similar cells that accommodate heating flues form as a battery. Coal, crushed to 80 % minus 3 mm with a top size of 15 mm, is loaded along the top of the ovens using a charging car on rails and is leveled by a retractable bar.
The operation of each oven is cyclic, but the battery contains a sufficiently large number of ovens to produce an essentially continuous flow of raw coke oven gas. The individual ovens are charged and emptied at approximately equal time intervals during the coking cycle. Coking proceeds for 15 hours to 18 hours to produce BF coke. During this period, VM of coal distills out as COG. The time of coking is determined by the coal blend, moisture content, rate of under firing, and the desired properties of the coke. When demand for coke is low, coking times can be increased to 24 hours. Coking temperatures generally range from 900 deg C to 1100 deg C and are kept on the higher side of the range to produce BF coke. Air is prevented from leaking into the ovens by maintaining a positive back pressure in the collecting main. The ovens are maintained under positive pressure by maintaining high hydraulic main pressure of around 10 mm water column in batteries. The gases and hydrocarbons which evolve during the thermal distillation are removed through the off take system and sent to the by-product plant for recovery.
The coking is complete when the central temperature in the oven is around 950 deg C -1000 deg C. At this point the oven is isolated from hydraulic mains and after proper venting of residual gases, the doors are opened for coke pushing. At the end of coking period the coke mass has a high volume shrinkage which leads to detachment of mass from the walls ensuring easy pushing. Coking takes place in completely sealed ovens, and when the carbonization is completed the oven doors are opened and a ram on one side pushes the red-hot coke into a quenching car.
The by-product gas and tar vapors leaving the coke oven undergo a separation process to remove the tar from the gas. The gas then is treated to recover NH3, as ammonium sulphate, while the tar is fractionated by distillation into three oil cuts, which are designated as light, middle (or tar acid), or heavy oil. The gas, mainly a mixture of H2 and CH4, has a good calorific value and is used as a fuel.
In case of non-recovery/heat recovery coke ovens, the heat energy of flue gases is recovered in the form of steam. In the process of coke making in the non-recovery ovens, volatiles evolved during coal carbonization are not recovered as by-products but are burned in the oven itself in the presence of controlled quantity of air and the heat of the volatiles of evolving gases is utilized for coking of the coal mass into coke and thus no external heating is needed. The higher level of heat importantly is used to break up the potentially polluting hydrocarbons into the constituent combustible compounds and to burn them thus avoiding the potentially hazardous pollution. The heat consequent to combustion is only partially utilized during the process and the balance heat in the waste flue gas is recovered for energy generation.
Non-recovery ovens are generally of horizontal design and operate under negative pressure unlike by-products ovens which operate under positive pressure. Primary combustion air, introduced through ports in the oven doors, partially burns directly the volatiles (Including tar and benzol) in the oven space above the coal. This generates the heat needed for the process. The mixture of the crude and the waste gases is led through the vertical ducts in the side walls to the heating flue system under the oven sole. Secondary air is introduced into the sole flues, which runs in the serpentine fashion under the coal bed and completes the combustion of the gases. The design of the flues and the control of the air flow allow the coking rate at the top and bottom of the coal bed to be equalized. Due to the temperatures generated, all the hydro-carbons and by-products are burned within the oven. The time of coking varies from 48 hours to 72 hours depending upon the design of the non-recovery coke ovens. Hot gases pass in a waste tunnel to heat recovery steam generators (HRSG), where high pressure steam is produced which is normally utilized for power generation.
Process of carbonization
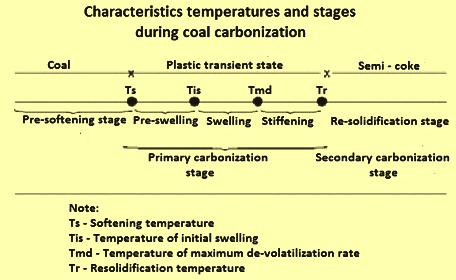
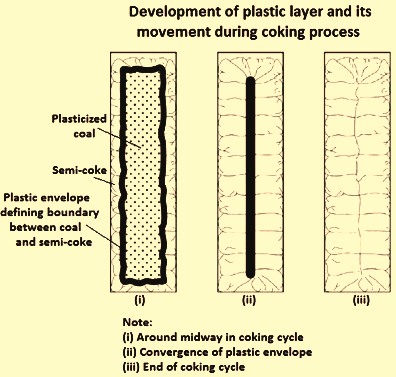
When coal is charged into a hot coke oven, that portion of the coal, which is directly in contact with the walls, is heated very rapidly. When its fusing is reached, the thin layer of heated coal softens and fuses. Destructive distillation reactions proceed rapidly in the plastic layer, with abundant evolution of volatile products. The gas and condensable vapours are entrapped in the plastic mass and, as they expand, tend to swell it. As the reactions proceed and as the temperature of the fused zone increases, the plasticity of the charge begins to decrease. With continued heating and evolution of gas the fused layer gradually re-solidifies to the typical, cellular, coke structure. The characteristics temperatures and stages during coal carbonization is shown in Fig 1, and the development of the plastic layer and its movement during coal carbonization process is shown in Fig 2.
Fig 1 Characteristics temperatures and stages during coal carbonization
Fig 2 Development of the plastic layer and its movement during carbonization process
The coke left after solidification of the plastic zone still contains considerable amount of VM, as its temperature is raised still higher, the destructive-distillation reactions continue with evolution of gas and a little tar. The final reactions which take place in the coke appear to be largely splitting off H2 from the extremely complex, high-molecular-weight hydrocarbons of which it is composed. With increasing temperature the coke tends to pull away from the oven walls, and shrinkage cracks develop, which run from the oven walls inward toward the centre of the coke mass. The two plastic zones move slowly from the opposite walls of the oven towards each other and finally meet at the centre of the oven. The junction of the zones appears as a vertical crack running lengthwise through the oven at the centre of the charge. When an oven is pushed, the coke divides vertically at this crack.
As the plastic zones move inward, their rate of travel tends to decrease because of the increasing distances through which the heat is to be conducted. Increase in the sensible heat carried off by the gas passing upward through the coke, and any heat absorbed in the cracking reactions which occur in the gaseous, also tend to slow down the rate of travel of the zones. The temperature and behaviour of the charges which are in the different zones of oven are different at different coking times.
The coal to coke transformation takes place as the coal is heated. When the state of fusing is reached, the layer of heated coal softens and fuses. From around 375 deg C to 475 deg C, the coal decomposes to form plastic layer. Destructive distillation reactions proceed rapidly in the plastic layer with the evolution of volatile products. At around 475 deg C to 600 deg C, there is a marked evolution of tar, and aromatic hydrocarbon compounds. The gas and condensable vapour are entrapped in the plastic mass and, as they expand tend to swell it. As the reactions proceed and as the temperature of the fused zone increases, the plasticity of the coal decreases. With continued heating and evolution of the gas the fused layer gradually re-solidifies into semi-coke having typical, cellular coke structure. The coke at this stage still contains substantial amount of VM. As the temperature increases further beyond 600 deg C, the destructive distillation reaction continues with the evolution of gas and a little tar. The coke stabilization takes place as the temperature increases from 600 deg C to 1100 deg C. This is characterized by contraction of coke mass, structural development of coke and final H2 evolution. At this stage the final reactions take place. These reactions split off H2 from extremely complex, high molecular weight hydro- carbons. With increasing temperature, the coke mass shrinks with the development of shrinkage cracks.
Mechanism of carbonization
During the process of carbonization, powdered coal is transformed into a porous, fissured, silver-black solid, coke. Microscopically, coke consists of a solid matrix, organic and inorganic inclusions in the matrix, pores and micro-fissures. The processes of the development of the porous structure and the micro-texture of coke take place essentially within the plastic range. The structure formed in the coke by the gas bubbles occupies almost half of its volume and influences two properties of coke, the mechanical strength and the bulk density.
The solid material forming the pore walls consists of optically-anisotropic entities which are usually observed using polarized light microscopy (PLM). The coke micro-texture influences the coke properties which are essential for its use in the BF.
During the carbonization process, metallurgical (coking) coals soften to become plastic, decompose, swell, agglomerate and finally re-solidify. The plastic temperature range is generally in the range of 350 deg C to 500 deg C. Thus in the carbonization process, two vertical plastic layers are formed parallel to the heating walls which proceed towards the centre where they coalesce (Fig 2). During carbonization some coals generate internal gas pressures and exert measurable wall pressures, sometimes dangerously high, on the oven walls.
During the process of carbonization, two processes occur during the plastic temperature range. The pore structure of the coke develops and the coal C becomes organized into graphite type layer planes the ordering of which results in the optical anisotropy of the coke. The variations in size of the anisotropic units give rise to textural components in the coke. The proportions of the various components present being the textural composition of the coke.
There are several studies regarding the mechanism of carbonization during high temperature carbonization process, and some hypotheses of coal carbonization mechanism have been given. Two of them are plastic carbonization mechanism and mesophase carbonization mechanism.
In the plastic carbonization mechanism of coal, it is considered that there are three continuous reactions of conversion from coals into cokes through the plastic phase. When coking coals are heated up above 350 deg C in the absence of air, the organic matter of the coal begins thermal decomposition. The mixtures of the gas, liquid and softened coal, which are the thermally decomposed from of coal, are called a plastic mass. The coking coals soften, melt, fuse, swell, and join together within a plastic stage. When the temperature is raised to 450 deg C to 550 deg C, a part of the plastic mass is evolved in gas and condensable vapour and the other part re-solidifies to the semi-coke. With further increasing in temperature above 550 deg C, the organic matter of the semi-coke decomposes and condenses further, the CH4 and H2 are evolved, the C lamellae of the semi-coke increase continuously, the coke is then finally formed. The two main stages which are converted from coals into coke are the carbonization phase of producing a plastic mass and the shrinking phase of the semi-coke.
In the carbonization mechanism by mesophase, the formation of anisotropic C from the isotropic melt of coal, pitch or selected model organic compounds are first attributed to the development of a distinctive phase of liquid crystals. When the coal is carbonizing, the plastic mass of optical isotropic is first formed, and then forms lamellar nematic liquid crystals gradually. This polymeric phase is termed mesophase. That is the intermediate phase between the isotropic fluid coal and the solid anisotropic semi-coke which is ultimately formed from the mesophase, and has properties of intermediate between solids and liquids. If the fluidity of the intermediate phase is sufficiently high, mesophase coalesce immediately into a single larger unit. Over a range of increasing temperature, mesophase is formed continuously, grows in size and ultimately touches each other. Thus the mesophase can solidify and convert from coal into optical anisotropic textures of coke.
A significant development in understanding carbonization process has been made with the discovery of mesophase in the plastic stage of carbonization leading to graphitizable carbons, as observed by optical microscopy. The development of spherical mesophase particles from an isotropic mass and their progressive growth and coalescence eventually to form anisotropic structures is well established for pitch-like precursors. Basically, during the carbonization process, de-hydrogenative polymerization of aromatic molecules occurs, with a consequential increase in average molecular weight. The final coke structure is related to the properties of mesophase at the time of solidification and these, in turn, are dominantly dependent upon the chemical properties of the parent material. Coke quality improvements are dictated by the quality of the parent feedstock which predetermines the optical texture of the resultant coke.
In contrast to pitch-like materials, carbonization of coal produces mesophase in the form of much distorted spheres which do not show observable coalescence because of their high viscosity. These differences in behaviour can be attributed to inhibiting effects of elements such as N2 (nitrogen), O2, and S (sulphur) and to the influence of particulate inert matter in the coal.
The mechanism and the principal factors influencing the formation of pores in semi-coke have been the subject of various studies. These studies have shown that the pore structure of coke is largely determined within the plastic temperature range of the carbonization process. During the studies, it has been observed that initially pores appeared in large particles at a temperature near the softening point, while the medium size particles became porous at higher temperatures. No pore formation has been detected at any temperature within particles less than 125 micro meters (microns) in size. An increase in temperature induced an increase both in the number and the size of pores. More particles have been observed to have pores and the large particles became multi-pored. With increasing temperature particles became more rounded and swelled into the inter-particulate voids.
In the case of coals of zero fluidity, the above mentioned stage marked the end of the observed changes. However, for coals of higher fluidity, an increase in temperature, reaching eventually the temperature of maximum contraction, results in the continued swelling of the larger particles and the concentration of the small ones in the diminishing void spaces. The swelling of the more fluid coals continues until all the small particles are incorporated within the expanding cell walls of the larger particles thus leading to the loss of their separate identity. Above this temperature of complete fusion, the mean pore size increases reaching a peak value before it finally falls to a size which is practically unaltered at higher temperatures. The increase in the number of pores with temperature is interrupted by a transitional minimum at the temperature which corresponds to the maximum pore size. The size of these large pores is reduced before the re-solidification temperature, thus leaving a more coherent structure. The growth of small almost spherical pores and fusion of the matrix near this temperature generally results in the formation of interconnected pores.
Above the re-solidification temperature insignificant changes are observed apart from a slight reduction in the mean pore size. The compaction of the completely fused structures which occurs near the re-solidification temperature has aroused a great interest. The explanation for this is that the large expansion of the slightly colder zone can press the compacting layer against the more rigid semi-coke. An inter-connection of pores possibly occurring at this stage can facilitate this process. This explanation is further supported by the observation of a development of anisotropy in pore shape, with the largest dimension lying along the temperature isotherms.
A further study of the influence of charge density and particle size on the pore structure development shows that both mean pore and pore-wall sizes attain maximum values within the plastic zone. The new findings are explained by the suggestion of two further processes namely (i) the rupture of some thin pore-walls during the post fusion expansion stage, and (ii) a secondary pore nucleation occurring at higher temperatures during the compaction process. It has been shown that an increase of the charge density reduces the porosity, mainly the mean pore size of the resultant coke, whereas the mean pore-wall size shows negligible variation. The effect of charge density is attributed to the restriction of the expansion along the horizontal direction which is perpendicular to the oven wall. No evidence is found for any systematic variation in the coke pore structure resulting from variations in the coal particle size distribution within the range considered.
An investigation has been carried out to study microscopically the transformation of coal to coke. In this investigation the study has been done regarding the morphology of the plastic layer as a function of coal rank. It has been observed that for coals with VM content in the range of 25 % to 30 %, the first step in the transformation is the formation of a continuous medium from the viscous deformed coal particles which is characterized by the absence of bubbles. The second step is the devolatilization which is obvious by the presence of a highly porous zone. The conclusion of the study has been that the formation of the pores begins suddenly when the borders of individual particles are not identifiable. In addition to the melting zone with lack of pores there is another one rich in pores with very thin walls which have been described as a froth zone.
In similar studies, a difference between the high and medium rank coals has been noticed. It has been observed that, in the case of medium VM coals, during re-solidification the compaction of the bubbles dominated their formation while the opposite happened during the preceding zone of maximum fluidity. There existed a mass transport in the plastic layer from the region of maximum fluidity to the semi-coke which is expressed in the high porosity measured in the centre of the plastic layer. In the case of high rank coals no similar maximum has been observed and this has indicated that for these coals a different mechanism for the elimination of bubbles may exist.
Carbonization pressure
The carbonization pressure developed during carbonization is expressed as a force exerted on the wall and is measured by means of a suitable device and is called the wall pressure. It is a phenomenon which has become important with the use of the double-heated wall, vertical, slot-type coke ovens. In the round beehive ovens, the coal can freely expand upwards and thus the swelling of the charge is accommodated by this free expansion. On the contrary in slot-type ovens the expansion of the coal horizontally to the heated wall is restricted.
It is established that some coals can damage coke oven walls because of either excessive pressure developed during carbonization or insufficient coke contraction at the end of the carbonization process. This problem has lately become a matter of importance due to coal preheating and the widespread acceptance of tall batteries which increase the bulk density of the coal charge, so affecting coking pressure, contraction and coke oven life. The effect of pressure developed during carburization of coal in the form of undesirable occurrences of distorted walls is more visible in taller ovens.
Many factors have been found to affect the magnitude of carbonization pressure. These factors can be separated into three broad categories specifically (i) inherent characteristics of the coal, (ii) coal preparation and physical properties, and (iii) oven operating conditions.
Whether a pressure is excessive or it does not depend not only on what pressure is exerted but also on what pressure the oven wall can withstand. Hence, various efforts have been made to assess the strength of the coke oven walls. The wall strength requirement is governed largely by the peak unbalanced coking pressure which is exerted on the walls during the carbonizing process. These unbalanced pressures cause wall bending in the horizontal direction which needs to be stabilized by the vertical gravity loading, including the weight of the roof and the wall, because the joints in the wall have no consistent tensile strength.
A very Iow limit restricts flexibility in choosing coal sources, coal blends and carbonization conditions. In a study, which compared the results of several hundred coals carbonized in a movable-wall oven and, taking into account the behaviour of these coals in commercial ovens, established the following safety limits for coals carbonized in coke ovens.
- Coals developing a pressure greater than 0.14 kilograms per square centimetres (kg/sq cm) are dangerous.
- Coals developing a pressure greater than 0.1 kg/sq cm can be dangerous when carbonized regularly in ovens taller than 3 metres.
- Coals which give pressures less than 0.1 kg/sq cm are safe.
The study has shown that an elastic deflection of commercial oven walls up to 1.25 mm can take place without the appearance of cracks. Beyond that point further deflection does not occur readily and cracking takes place.
In the 1960s and 1970s, the construction of tall coke ovens (height 6 m and above) became prevalent. These ovens are being operated under the assumption that coking pressures under 0.14 kg/sq cm are safe. The result was that in some cases these ovens sustained serious, early refractory damage. It has been reported that a 6 m tall battery had suffered progressive damage and had to be shut down after less than five years of operation. The investigations to determine the causes of the premature failure included a structural analysis of a 6 m tall wall. A mathematical study has been carried out of a 6 m tall oven wall subjected to pressure from one side. From this analysis the unbalanced lateral pressure which can cause collapse has been calculated to be just above 0.12 kg/sq cm. By taking into account the recommended live load factor of 1.7 as well as serviceability relative to cracking, it has been recommended that the allowable unbalanced lateral pressure is not to exceed 0.07 kg/sq cm.
Leave a Comment